
Get Instant Solution By an Expert Advisor
(4.8)
CDSCO Medical Device Registration has been required for Manufacturers, importers, wholesalers, and distributors of Medical Devices and diagnostics. Obtain CDSCO Medical Devcie (MD) Online Registration with the support of Agile Regulatory a team of experienced Medical Devcie Professionals.


10000 +
Projects Completed for Our Respected Clients.
15 +
Years Experienced Advisors in Indian Compliance.
98.9%
Project Delivery Ratio for Our Valuable Clients.
99.9%
Satisfied Customers All Over India.
Medical Devices have become more than just a necessity for human lives as they offer prevention, analysis, and speedy recovery from life-risking diseases. However, earlier there was a lack of regulations for medical devices giving rise to the manufacturing of sub-standard medical devices and equipment. However, the CDSCO launched the medical device registration scheme in the year 2006 for monitoring and regulation of medical device registration. The CDSCO is primarily responsible for registering medical devices, regulating new drugs and clinical trials, and setting standards for drugs.
It also coordinates the work of the state drug control organizations and offers expert advice in order to ensure that the Drugs and Cosmetics Act is consistently enforced throughout the nation. Any individual or organization involved in the production, import, sale, or distribution of medical devices must thus register their medical device through the CDSCO Portal.
CDSCO Medical Devices include any instrument/apparatus/appliance/ implant/material or another article, either used solely or in combination including any software or accessory designed by the manufacturer to be employed particularly for human beings or animals that don't attain the key proposed action in or on human body or animals through any pharmacologic/ immunological/metabolic means, but assists in its envisioned function:
Some of the CDSCO medical devices that need registration in India include spinal needles, cochlear implants, annuloplasty rings, tracheostomy tubes, dental implants, surgical sealants, heart valves, cardiac stents, orthopaedic implants, endotracheal tubes, etc.
Currently, there are two different categories of medical devices for Medical Devices Registration through the SUGAM portal which have been explained below:
Notified Medical Devices: As of now, the CDSCO authorities have notified a list of 30+ categories of medical devices that mandatorily require registration and even require prior approval from CDSCO to market such marketing devices in India. For such devices, there are separate forms for application and different documents required to be submitted to the approving authority.
Non-Notified Devices: Further, other non-notified devices have not yet been notified and regulated. Thus, the registration of such devices has been made voluntary under the CDSCO SUGAM portal.
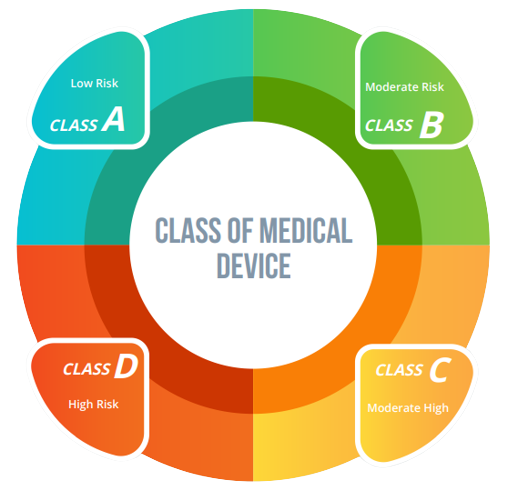
Class of Medical Device:
The list of individuals who can submit applications for CDSCO Medical Device Registration in India is as follows
Meets Significant Requirements of the Indian Market: In order to address the substantial need for accessible healthcare in India, the Indian authorities are progressively depending on international manufacturers to fulfil the considerable requirement. As a result, the implementation of the medical device registration scheme becomes valuable for ensuring control and uniformity in the quality of these products.
Registration Monitoring and Oversight: Following registration, the Drugs and Cosmetics Act mandates manufacturers and distributors to give precedence to adhering to the provisions of the Act. This involves conducting on-site evaluations of medical devices. Manufacturers have the opportunity to aid the audit team in identifying possible concerns, thereby averting penalties for violations through product registration. This process guarantees adherence to regulations and the safety of patients.
Legal Protection through Compliance: Relentless pressure to maintain medical device compliance has made manufacturers worry about preparing and distributing products according to prescribed guidelines, but with medical device registration, it becomes easier.
Opportunities for Higher Growth & Sales: The Indian government addresses escalating demand for medical devices by allowing overseas manufacturers to enter the Indian market, ensuring compliance with CDSCO guidelines, and maintaining a high-profit margin.
Improve Brand Identity: The Indian market has seen a shift in manufacturers' obligations to register their products, allowing them to explore the market dynamically. However, the steep penalties make it sense not to infringe on guidelines for profit.
|
Applicant Type
|
Device Class
|
Application Form
|
Form of License
|
|---|---|---|---|
|
Importer
|
A, B, C, D
|
MD-14
|
MD-15
|
|
Manufacturer
|
A, B
|
MD-3
|
MD-5
|
|
Manufacturer
|
C, D
|
MD-7
|
MD-9
|
|
Loan Licence
|
A, B
|
MD-4
|
MD-6
|
|
Loan Licence
|
C, D
|
MD-8
|
MD-10
|
Packaging description comprising pack sizes.
Copy of the Site Master File & Plant Master File.
Labelling details conforming to Drugs & Cosmetics Rules, 1945.
Recommended storage conditions, Brief description of the device.
Testing facilities are available in the manufacturing units for testing.
Details of the Product such as Brand or Proprietary name, Device category.
A detailed explanation of the manufacturing process of the devices to be manufactured.
List of accessories & other devices or equipment to be used in combination with the device.
Copies of ISO or any other certifications, if required, obtained by the firm for its manufacturing premises.
Details of the standards followed by the company for product examination & Good Manufacturing Practices.
Summary of the company's plan, a device to be manufactured, their viability & other important profiles.
Qualification, experience & name of technical staff under whose supervision the devices will be manufactured.
Method of use and intended use, Specification of the materials used, Variations in style, size, or shape of the device, if applicable.
Details of the applicant entity like names, addresses of the company's directors and addresses of the manufacturing units and registered offices of the Manufacturer.
Step 1: Product Verification: The initial stage in the process of importing a medical device involves verifying whether it necessitates registration under the Medical Device Rules of 2017 and determining its classification as either a regulated or unregulated product.
Step 2: Portal Registration: After this, navigate to the online CDSCO SUGAM platform and initiate the signup procedure to acquire login credentials for website access.
Step 3: Application Submission: Submission of an application for a manufacturing license for these specified devices to the SLA using Form 27, and for the CLA through Form MD-3 or MD-7, alongside the necessary fee on the online portal.
Step 4: Scrutiny of the Application: On receipt of the application, the approving body will scrutinize the application, conduct an inspection of the manufacturing premises, and perform tests on the medical devices.
Step 5: Grant of Certificate: After which, if the approving authority is of the view that the applicant's medical device in question is genuine and manufactured in line with the pre-specified guidelines, then it may grant the registration, followed by a notification to the applicant.
Obtaining the Medical Devices registration could be a bit challenging due to the complex process of the documents required and the processes involved. However, with the right amount of guidance and consultation, you could easily acquire Medical Device registration within the prescribed time. At Agile Regulatory we are committed to helping budding entrepreneurs by enabling them to acquire business licenses smoothly and carry on their business.

Get Instant Solution By an Expert Advisor
(4.8)
A nominated representative is qualified to apply for acquiring the Import License for Medical Devices in India. The representative should possess a valid license for manufacturing (intended for selling or distributing) or maintain a wholesale license in accordance with the directives outlined by CDSCO, as stated in Form 20B and Form 21B. This authorized representative has the potential to be either an individual or a business entity.
The Central Drugs Standard Control Organisation (CDSCO) under the Government of India, operates as the National Regulatory Authority which also acts as the Central authority responsible for granting and monitoring the medical devices manufactured or imported in India.
The certificate remains effective for 5 years starting from the registration date unless it is suspended or revoked by the DGCI authority for valid reasons.
Medical devices are divided into four distinct categories denoted as A, B, C, and D, while devices categorized as A & B could be understood as the ones with moderate risk whereas C and D encompass devices with elevated and extremely high levels of risk. Manufacturers producing A and B categories of goods shall be registered with the SLA whereas the C& D category of medical devices must be registered under the CLA.
The timeline for medical registration devices registration may take around 6-9 months to complete and accurate documentation and fees to obtain a registration certificate. Once obtained, the registration shall be valid for a period of 5 years and a renewal application needs to be filed needs to be submitted 6 months in advance of expiry.
In order to register medical devices in India, you first need to classify the device (Class A, B, C, or D), subsequently appoint an Indian Authorized Agent (in the case of foreign manufacturers/importers), make a complete technical dossier containing Device Master File (DMF), Plant Master File (PMF), and ISO 13485 certification, and lastly, file the application through the CDSCO Sugam online portal along with the fees as stipulated, which will be scrutinized by the State Licensing Authority (in the case of Class A/B) or Central Licensing Authority (in the case of Class C/D), involving possibly inspections, resulting in an import (MD-15) or manufacturing (MD-5/MD-9) license.
In India, medical devices are mainly approved by the Central Drugs Standard Control Organization (CDSCO), which is the national regulatory agency. The Central Licensing Authority (CLA) of CDSCO deals with the approval of imported medical devices (through MD-15 license) and manufacturing of Class C and D medical devices. The State Licensing Authority (SLA) approves the manufacturing of Class A and B medical devices and licenses for sale, stock, exhibition, or distribution (MD-42).
Any institution or individual interested in selling, stocking, displaying, or distributing medical devices (including in-vitro diagnostic medical devices) on a wholesale scale in India qualifies for an MD-42 license, provided they have conditions like suitable premises, well-trained technical personnel (who may be a graduate of a recognized university, a registered pharmacist, or an intermediate with experience in selling medical devices for one year) and adherence to Good Distribution Practices (GDP).
Proven 4-step Process: Consultation, Documentation, Submission, and Certification.
Startups to large enterprises, we deliver end-to-end solutions business compliance needs.

What our customer says about us
Fantastic support from the team. Their expertise transformed our approach, driving remarkable outcomes. A must-have partner for businesses seeking effective consulting solutions. Highly recommended.

Lavkush Sharma
KTPL Instruments
Agile Regualtory delivers exceptional solutions. Their insightful guidance streamlined our processes and boosted profitability. Highly recommended for businesses seeking expert consulting services to thrive.

Nitin Mukesh
Justrack IOT
Impressed by Agile Regulatory's expertise. Their strategic insights and practical solutions have elevated our business operations. A reliable partner for effective consulting services. Highly recommended for growth-focused businesses.

Pradeep Varma
Coaire Compressor
Extraordinary consulting services. Their insightful solutions and dedicated team reshaped our business, driving remarkable improvements. Highly recommend it for transformative results.

Bharat Bachwani
Easy Polymer
Incredible experience with Agile Regulatory. Their innovative strategies and expert advice revitalized our business model, resulting in impressive growth. Highly recommend their exceptional consulting services.

Atul Jain
Tarus International
Top-tier consulting! offered strategic solutions that revolutionized our approach. Their deep expertise and personalized guidance made a significant impact on our success. Highly recommend their services.

Paramjeet Singh
Anchor Weighing
Agile Regulatory exceeded expectations! Their tailored solutions, expertise, and proactive approach led to remarkable results. Highly recommend for businesses seeking impactful and strategic guidance.

Anshul Rathi
AM Capacitor
Outstanding service! delivered targeted solutions with professionalism and expertise. Their insights elevated our business strategies, resulting in noticeable growth. Highly recommended for exceptional consultation.

Shekhar Maurya
Imaxx Pro Aquistic