
Get Instant Solution By an Expert Advisor
(4.8)
MD 42 license is the official Medical Device Registration Certificate granted by the State Licensing Authority commonly know as FDA or Food Safety and Drug Administration. This certification authorizes retailers, wholesalers, and stockists to sell, stock, exhibit, or distribute medical devices, including In-Vitro Diagnostic Devices. It ensures that these entities comply with regulatory standards for the lawful handling and distribution of medical products.


10000 +
Projects Completed for Our Respected Clients.
15 +
Years Experienced Advisors in Indian Compliance.
98.9%
Project Delivery Ratio for Our Valuable Clients.
99.9%
Satisfied Customers All Over India.
Diagnosing and evaluating patients in this complex healthcare environment requires medical equipment and in-vitro diagnostics (IVDs). The demand for medical devices has increased rapidly due to the changing healthcare sector in India. The Indian government places regulations that organizations need to follow to ensure the safety, accessibility, and quality of medical equipment.
Obtaining a medical device wholesale license Form MD–42 becomes imperative. If you are a wholesaler, retailer, and stockiest of medical equipment, you should receive a registration certification from the state licensing authority on Form MD – 42. The MD 42 Registration Certification is essential from September 30, 2022.

The Central Drug devices and Control Organization or CDSCO monitors Indian medical devices (It is) regulated under the 1940 Drugs and Cosmetic Act and 1945 Rules. Also, the pharmaceuticals from India are registered through the CDSCO process. The body will permit the importing and manufacturing medical devices, cosmetics, and pharmaceutical products.
New Rules from the Department of Health and Family Welfare:
The Ministry of Health and Family Welfare and the Department of Health and Family Welfare have recently introduced a new regulation called Medical Devices (5th Amendment) Rules, 2022. This amendment has Section 12(1) and Section 33(1) of the Drugs and Cosmetics Act passed in 1940.
Some modifications have been made to the initial Medical Devices Rules issued in 2017, which took effect on September 20, 2022. The essential modifications include:
The specifics of Rule 87A are as follows:
This regulation seeks to rationalize and provide the legal framework for the selling and distribution of medical devices to meet the new standardization standards and necessary oversight for improving public health.
The process of getting a Form MD 42 licernse registration certificate is as follows:
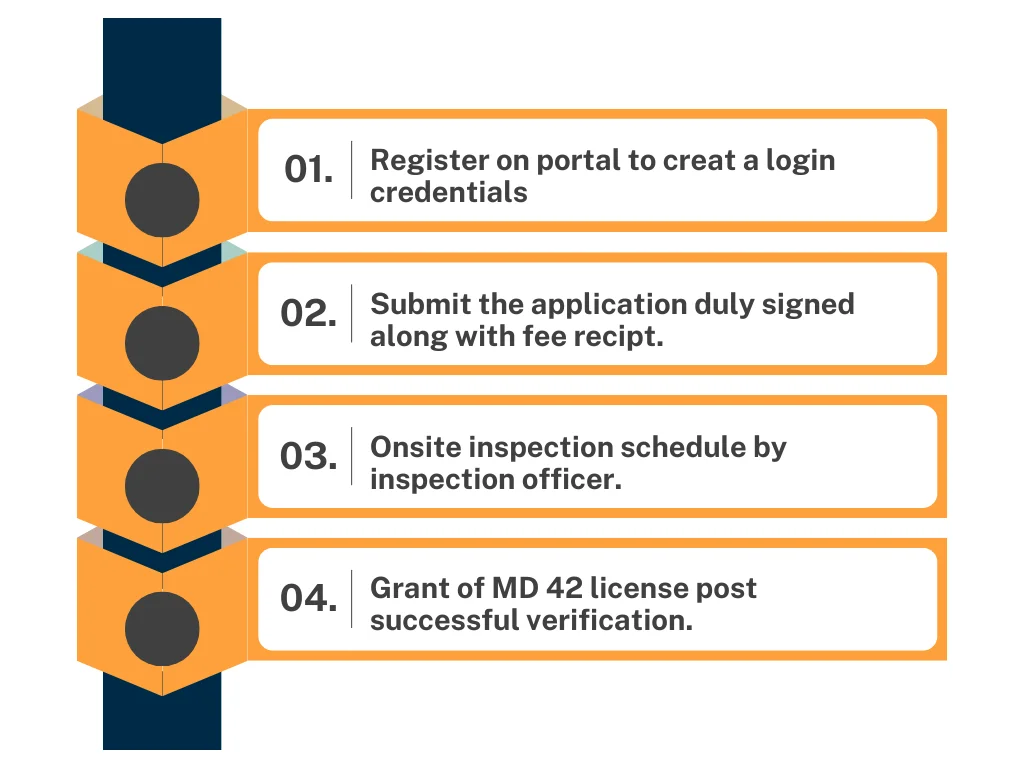
Step 1: Preparation Application: It initiates a comprehensive application with the business organizations that includes details of the business, ownership, structure, personnel, and storage facilities.
Step 2: Documentation: Organizations or individuals must submit address proof, identity, technical information, etc.
Step 3: Premises Condition: The premises where the medical equipment and IVDs are stored and distributed should comply with the regulations set by the CDSCO.
Step 4: Quality Management System :The applicant needs to set up a Quality management system (QMS) that includes details like managing, distributing, and storing medical equipment. The quality management system ensures compliance with quality standards and regulations instructions.
Step 5: Application Fees Payment: The applicant must pay a fee of Rs. 3000 at the time of submission of the application.
Step 6: Onsite Evaluation: After application submission, Department needs to evaluate the premises to check and read compliance with the rules and information given in the application.
Step 7: Grant of MD 42 License: After throughly evaluation of the application and premises an applicant will get the MD 42 License.
The authority will issue MD 42 certifications valid indefinitely, but the retention charges should be paid periodically, and obligations remain for five years until the issuance date. Note: The state government is not authorized to cancel the certificate. If retention changes are not paid within the deadline, then late fees of 2% of the retention changes until the payment is paid. It will be canceled unless it is paid within six months.
Suppose the certificate holder violates the regulations of a medical certificate or the state licensing authority. In that case, they may allow the certificate holder to show why they should not have their license suspended before suspension occurs. Upon suspension, the certificate holder should be provided written reasons for this action.
Appeal:
Agile Regulatory plays an important role as an MD 42 medical device wholesale license registration consultant in Noida. We offer a smooth registration process for obtaining a MD 42 license and ensuring compliance with local and international standards. Our expertise facilitates the efficient registration and licensing of medical devices, helping businesses navigate legal requirements, reduce delays, and boost market entry. By offering tailored advice and support, Agile Regulatory enhances operational efficiency and ensures adherence to regulatory frameworks, crucial for maintaining competitive advantage in the medical device industry.

Get Instant Solution By an Expert Advisor
(4.8)
The MD 42 license is granted by the State Licensing Authority once they review all necessary details. After confirming that the applicant has complied with all the regulations for medical devices, the license is issued in Form 42.
MD 42 license is issued by the State Licensing Authority to retailers, wholesalers, and stockists. This certificate allows them to legally sell, store, display, or distribute medical devices, including in vitro diagnostic instruments.
The MD 41 License is issued by the State Licensing Authority. They are responsible for reviewing and either approving or rejecting applications submitted in Form MD-41, which is used to apply for the registration certificate needed to sell, stock, display, or wholesale medical devices
In India, a Medical Devices Wholesale License is issued by the Central Drugs Standard Control Organization (CDSCO). To register specific medical devices with an Indian counterpart, the CDSCO uses the MD 41 form. This license is required for wholesalers who wish to distribute medical devices in the country.
To sell or distribute medical devices, you need to apply for a registration certificate by submitting Form MD-41 to the State Licensing Authority (SLA). Our team of experts is here to assist you through the entire process, making sure everything goes smoothly and without any issues.
The MD 42 license is mandatory for manufacturers, importers, wholesalers, and retailers involved in the manufacture, import, sale, and distribution of medical devices, both retail and wholesale.
Proven 4-step Process: Consultation, Documentation, Submission, and Certification.
Startups to large enterprises, we deliver end-to-end solutions business compliance needs.

What our customer says about us
Fantastic support from the team. Their expertise transformed our approach, driving remarkable outcomes. A must-have partner for businesses seeking effective consulting solutions. Highly recommended.

Lavkush Sharma
KTPL Instruments
Agile Regualtory delivers exceptional solutions. Their insightful guidance streamlined our processes and boosted profitability. Highly recommended for businesses seeking expert consulting services to thrive.

Nitin Mukesh
Justrack IOT
Impressed by Agile Regulatory's expertise. Their strategic insights and practical solutions have elevated our business operations. A reliable partner for effective consulting services. Highly recommended for growth-focused businesses.

Pradeep Varma
Coaire Compressor
Extraordinary consulting services. Their insightful solutions and dedicated team reshaped our business, driving remarkable improvements. Highly recommend it for transformative results.

Bharat Bachwani
Easy Polymer
Incredible experience with Agile Regulatory. Their innovative strategies and expert advice revitalized our business model, resulting in impressive growth. Highly recommend their exceptional consulting services.

Atul Jain
Tarus International
Top-tier consulting! offered strategic solutions that revolutionized our approach. Their deep expertise and personalized guidance made a significant impact on our success. Highly recommend their services.

Paramjeet Singh
Anchor Weighing
Agile Regulatory exceeded expectations! Their tailored solutions, expertise, and proactive approach led to remarkable results. Highly recommend for businesses seeking impactful and strategic guidance.

Anshul Rathi
AM Capacitor
Outstanding service! delivered targeted solutions with professionalism and expertise. Their insights elevated our business strategies, resulting in noticeable growth. Highly recommended for exceptional consultation.

Shekhar Maurya
Imaxx Pro Aquistic